Funding statement
This project is funded by the European Union’s HORIZON-EIC-2021-PATHFINDER CHALLENGES program under grant agreement No 101070939 and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 22.00198.
Disclaimer
Intrecom is funded by the European Union and the Swiss State Secretariat for Education, Research and Innovation (SERI). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Innovation Council and SMEs Executive Agency and SERI (granting authorities). Neither the European Union nor the granting authorities can be held responsible for them.
Objectives
INTRECOM’s aims to substantially advance BCI technology and validate its working principle in people with LIS in the home environment.
The specific objectives of INTRECOM are to:
1. Develop a safe and fully functional high-density BCI prototype device with 128 channels (4 mm thick, size 2-2.5 cm, skull-mounted), brain surface-lining electrode grids, and high-channel connectors that allows safe and high-quality 24/7 brain signal recording.
2. Develop decoding algorithms based on AI that can translate brain signals to real-time computer speech (written or audio) with less than 1 second delay, thereby restoring communication by the end of the project.
3. Implant the developed prototype device in 2 people with LIS and validate its working principle, including use in the home environment with initial basic functionality within weeks after implantation, and real-time intelligible speech decoding by the end of the project.
4. Gather an unprecedented wealth of brain data through the in vivo research results. Data will improve INTRECOM’s hard- and software, speech translation algorithms, and functionality and use of the device. Openly shared, they will advance scientific knowledge of human brain function, BCI, and therapeutic opportunities, and provide novel real-time decoding principles for future closed-loop neurostimulation applications.
5. Show acceptability by participants, caregivers, and health care professionals by the end of the project.
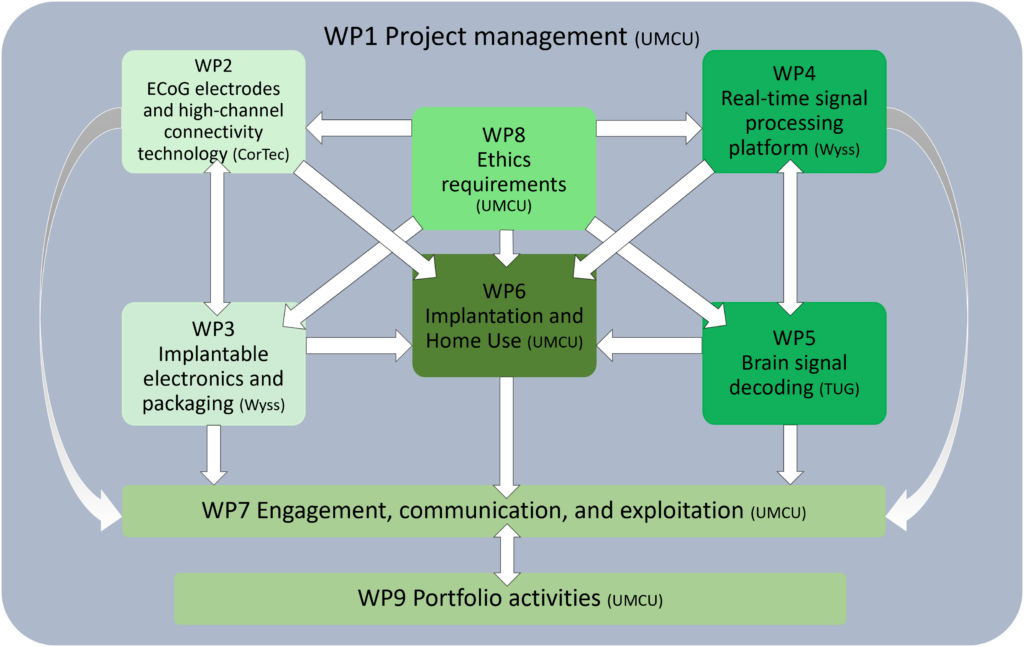
Work Packages
WP1 – Project management
Lead beneficiary: UMCU
WP1 will coordinate and manage the project in an efficient and effective manner, by ensuring the achievement of the project’s objectives and outputs on schedule and within budget, and by conducting good and effective project administration, and legal, financial, and data management. In addition, WP1 aims to liaise and cooperate with other successful projects within the Pathfinder portfolio.
WP2 – ECoG electrodes and high-channel connectivity technology
Lead beneficiary: CORTEC
WP2 will enable reaching INTRECOM objective 1: Develop a safe and fully functional high-density BCI prototype device.
Within WP2, we will develop state-of-the-art hardware applications, namely, 1) proprietary micro-laser fabricated multi-channel silicon rubber ECoG electrodes with customized spatial arrangements and sizes of electrode contacts (“AirRay” electrodes), 2) novel, extremely high-channel hardwired connectivity technology to connect the electrode cables to the electronics unit, and 3) high-channel count reversible implantable connectors.
WP3 – Implantable electronics and packaging
Lead beneficiary: WYSS
WP3 will enable reaching INTRECOM objective 1: Develop a safe and fully functional high-density BCI prototype device.
WP3 aims to develop a fully implanted, wirelessly powered and telemetered 128-channel neural interface by leveraging the current design and electronics of the WYSS BCI technology ABILITY and connect it to the ECoG grids developed in WP2. The device will be thoroughly bench tested and tested preclinically in a sheep model to ensure safety and functionality prior to implantation in humans. The novelty of the device comes from 1) The interface enabling wireless power transfer and management, 2) Optical transfer of data through 4 small windows, 3) Magnetic alignment of device and headpiece with minimally invasive surgically removable magnets that enable MRI compatibility, and 4) The fully implantable nature of the device ensuring no skin breach is necessary for data collection.
WP4 – Real-time signal processing platform
Lead beneficiary: WYSS
WP4 will enable reaching INTRECOM objective 2: Develop decoding algorithms based on AI.
NeuroKey is the proprietary WYSS real-time neural signal processing platform. It has been successfully used and CE Marked as Class A software as a medical device to enable communication with people with ALS in a locked-in state. In WP4, NeuroKey will be upgraded and its functionalities expanded to enable brain-to-speech communication at home. This will include developing a processor for the 128-channel ECoG signals and implementing algorithms developed by UMCU and TUG toward real-time speech decoding (WP5) from relevant brain areas. To accomplish this, WP4 will develop new data analysis, signal processing, and feature extraction functionalities based on AI. In addition, WP4 will develop a user interface for the end user.
WP5 – Brain signal decoding
Lead beneficiary: TUG
WP5 will enable reaching INTRECOM objective 2: Develop decoding algorithms based on AI.
WP5 will use two different approaches for decoding brain signals – attempted speech and attempted hand movement – as the implanted electrode grids will be positioned over the face and hand sensorimotor areas.
From the electrode grid placed over the hand area, we will decode basic communication abilities that participants can use quickly after implantation. We will start by developing and implementing a basic click function. In the course of the project, complexity and degrees of freedom will be gradually increased. Based on the detection and classification of different hand movements we will implement a system that allows the participant to select the direction of a cursor movement to select an item of choice.
For decoding of attempted speech, we target the sensorimotor face area. We pursue three different conceptual approaches to achieve satisfactory speech decoding (for real-time communication) that reflect different perspectives on speech: 1) speech as a series of discrete phonemes, 2) speech as an acoustic signal and 3) speech as a product of articulatory movements.
WP6 – Implant and home use
Lead beneficiary: UMCU
WP6 will enable reaching INTRECOM objectives 3 and 4: Implant the developed prototype device and Gather an unprecedented wealth of brain data.
Two people with late-stage ALS will be implanted with the BCI device, one at UMCU and one at TUG academic hospitals. When the participant has recovered, our research team will regularly visit the participant to train and test the system. Participants will receive home use equipment (antenna, signal acquisition unit, decoding and visual feedback tablet) programmed to accommodate basic control over a cursor (‘brain click’). During the research iterations, the decoding software will be continually updated to accommodate the advances made in decoding algorithms, with ample input from participants and their caregivers to ensure user satisfaction. With each satisfactory achievement in the speech decoding strategies (WP5), the home use system will be expanded with the new features and uses (WP4), and performance during home use thereof is monitored (preserving user privacy).
WP7 – Engagement, communication, and exploitation
Lead beneficiary: UMCU
WP7 aims to make the most of the results of INTRECOM. The tasks of WP7 are therefore to plan and execute dissemination and communication activities and to develop scenarios for economic exploitation of the developed device and its components. WP7 is also responsible for the engagement of the implanted participants in the ongoing research and to involve end-user representatives, such as patient organizations, for advice regarding key decisions in the project. In the end phase of the project, UMCU, together with all partners, will organize a symposium and workshop on implantable BCI for LIS, to which all relevant stakeholders will be invited.
WP8 – Ethics requirements
Lead beneficiary: UMCU
WP8 sets out the ‘ethics requirements’ that the project must comply with.
WP9 – Portfolio activities
Lead beneficiary: UMCU
WP9’s objectives are to strengthen the prospects for successful project completion and performing the initial steps of transition towards market by developing synergies and collaborations among the projects funded under the 2021 EIC Pathfinder challenge call – Tools to measure and stimulate activity in brain tissue (HORIZON-EIC-2021-PATHFINDERCHALLENGES-01-02), steered by the Programme Manager (PM) in charge of the portfolio of projects (Portfolio) generated by the call.
Timeline
December 1, 2022: Start of INTRECOM
During Year 2:
- Intracranial hardware safety tests passed
- Ethical approval obtained
- To-be-implanted hardware ready
During Year 3:
- Participant recruitment finished; informed consent obtained
- First version of decoding software operational
- Participants successfully implanted
November 30, 2026: End of INTRECOM; Participants can use speech system at home

